Product X works as a masonry sealer, but what are the long-term ramifications of using Product X on masonry buildings? Masonry sealers come in a wide variety of formulations, but how do the various chemical compositions react to environmental conditions and what affect does the formulation have on the masonry? Most masonry waterproofing sealers specified by architects and conservators, installed by contractors, and requested by property owners are based on Silicone (═Si═ ) chemistry. There are three popular groups of silicone based materials being used as waterproofing materials: 1) silicates, 2) the group of silane, siloxane, siliconate; and 3) silicones. Silicates, similar to Product X, provide waterproofing properties by filling the pore structure of building materials with silicon dioxide (SiO2) precipitation. Common silicates are sand, Portland cement, and other natural occurring minerals. Silanes, siloxanes and siliconates provide waterproofing properties by bonding with the substrate. They are often referred to as penetrating sealers. Silicones do not form chemical bonds with the substrate. Silicones provide waterproofing properties by forming a non-bonded film. Such products are labeled as thin-film sealers.

SILICATES
Silicates are most commonly used in crystalline type water proofing agents for concrete. Their use is generally focused on concrete substrates. However, it is known that strongly alkaline, aqueous solutions of methyl silicates can be used to impregnate masonry. Such solutions often depend upon caustic soda for their alkalinity. Impregnation of masonry with such solutions is often disadvantageous, however, particularly due to the high alkalinity. For example, the high caustic soda content of the solution will cause a gradual removal of the organosilicon compounds from the interstices of the masonry by chemical combination with the surfaces of the masonry surrounding the interstitial voids. Moreover, the caustic soda solution reacts with carbon dioxide or other acidic components of the air which gives rise to salting out and the formation of efflorescence on the masonry. (1)
SILANE, SILOXANE, SILCONATES
Silane, siloxane, silconates are penetrating type of sealants. Their effectiveness is dependend on the porosity of the substrate and the dosage of repellant applied. Each manufacturer will have unique requirements for the application and dwell time of their sealer. Silanes and siloxanes form a chemical bond with siliceous containing materials. Silanes and siloxanes go through three reactions when applied to a masonry surface: hydroloysis, condensation, and bonding. During the condensation phase, the moisture vapor transmission rate is critical to preventing moisture accumulation behind the sealer layer.
With penetration type sealers, it is critical to the longevity of substrate (masonry) that the moisture vapor transmission of the sealer is actually known. There has been very little third party testing of vapor transmission and each product manufacturer provides varying ways of testing transmission. In addition, the active ingredient content of the sealer formulation and the coverage rate will greatly affect the moisture vapor transmission. In other words, performance in the field will vary greatly from highly controlled laboratory testing.
Siliconates are water soluble and they impart water repellency on porous surfaces. A drawback to using diluted siliconate solution for waterproofing applications is that siliconates react with carbon dioxide and carbonatious matters present in the substrate to form a water repellent, water-insoluble, white colored precipitate. This white layer may become quite visible and require aggressive removal procedures resulting in objectionable appearance or scarification of the surface during removal processes.
SILICONE
The effectiveness of silicone sealer depends on the alkyl group used (which directly influences its resistance to alkaline conditions), the amount of exposure to ultraviolet light and the level of moisture in the masonry when the silicone is applied. (2)
The proliferation of masonry coatings on the market, and the continued pervasive use of the coatings, requires the architect, engineer, contractor, and conservator become more knowledgeable on the wide variety of coating formulations, the continued evolution of those formulations, and understand both the right application of the product and potential detrimental effects of using the wrong product on historic substrates.
CASE STUDY: WASHINGTON STATE UNIVERSITY, DUNCAN DUNN HALL
In preparation of a major renovation, Peter Meijer Architect, PC was retained in 2010 to conduct a general exterior condition assessment of Duncan Dunn Hall on the campus of Washington State University, Pullman, Washington.
Duncan Dunn was constructed in 1926 as a women’s dormitory for Washington State University, then named Washington State College. It is located in the heart of the WSU campus, facing north towards Linden Avenue. First known as the “New Dorm,” the building cost $150,000.00 to build at that time, and could house 140 students. The architect, Stanley Smith, was the head of the department of architectural engineering and was also the official University Architect.

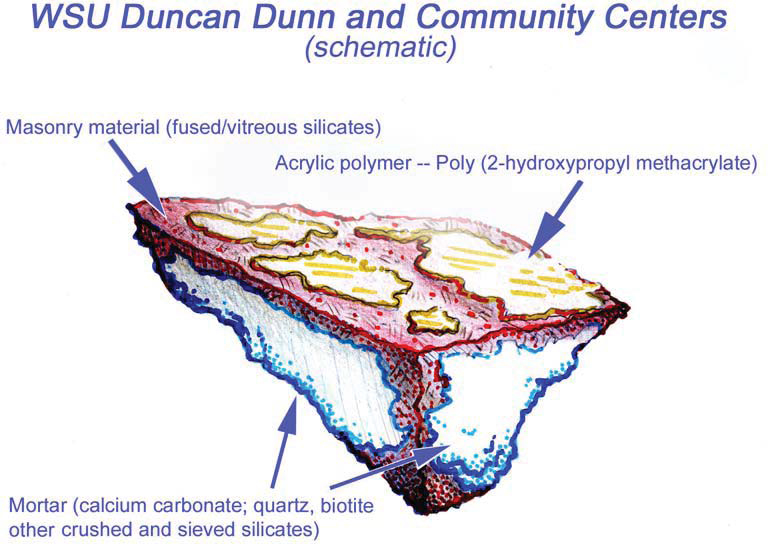
The predominant material present on Duncan Dunn is a solid brick unit, brownish red in color, and approximately 8” x 3 7/8” x 2 3/8” in size. At the time of assessment the brick had a very prominent unsightly, white coating over 60% of the masonry facades.
 Believing the white haze was a result of UV degradation of a masonry sealer, PMA conducted Reunion Internationale des Laboratoires D’essais et de Recherches sur les Materiaux et les Constructions (RILEM) tube tests of water absorption on the exterior brick on Duncan Dunn Hall. The area of brick chosen for the test was out of direct sunlight to avoid affecting the results and was conducted during dry weather. No movement of the water over a 45 minute period was recorded during the test. Masonry units, even those constructed with high quality clays under controlled firing conditions will absorb some water. The results of the field test on Duncan Dunn, along with the white surface haze, reinforced the assumption of the presence of a masonry coating.
Believing the white haze was a result of UV degradation of a masonry sealer, PMA conducted Reunion Internationale des Laboratoires D’essais et de Recherches sur les Materiaux et les Constructions (RILEM) tube tests of water absorption on the exterior brick on Duncan Dunn Hall. The area of brick chosen for the test was out of direct sunlight to avoid affecting the results and was conducted during dry weather. No movement of the water over a 45 minute period was recorded during the test. Masonry units, even those constructed with high quality clays under controlled firing conditions will absorb some water. The results of the field test on Duncan Dunn, along with the white surface haze, reinforced the assumption of the presence of a masonry coating.
Communication with WSU personnel and their internal research surmised that “the building may have had a sealer put on after the original construction. [WSU cannot verify the application through original records] but do know [that a sealer] was not used on a regular basis after [construction completion.] Back in the 70’s some “miracle sealer” of some sort was introduced on Campus and used at a few locations. Duncan Dunn Hall was among the buildings [receiving masonry sealers.] Today you can see the remnants of this as a white powdery surface that almost looks like efflorescence. [WSU] does not know the name of [the sealer] product.”
To confirm the presence of the sealer, PMA conducted lab testing via polarized light microscopy (PLM) episcopic microscopy, capillary fusion and Fourier-transform infrared spectroscopy (FTIR) per ASTM D1245 and E1252, respectively. FTIR indicated the material to be Poly(2-hydroxypropyl methacrylate), an initially water-miscible acrylic polymer that in these samples is at present very brittle and sloughs rather easily.Testing confirmed the presence of a “water-miscible acrylic polymer”. Due to chemical breakdown under UV, the chalky coating remaining on Duncan Dunn is no longer soluble in water.Because of the insoluble nature of the white haze, low pressure hot-water cleaning methods would not be successful. PMA recommended the Rotec Vortex cleaning system using a mirco-abrasive mixture of dolomite, water, and air. Ultimately this removal processes was successful with no damage to the masonry surface.
(1) Patent application for new formulation of sealers. (2) Types of Masonry Water Repellents, GSA web site. Information derived from ProSoCo Inc. product literature.
Written by Peter Meijer AIA, NCARB / Principal